Chemistry 12
A Greek philosopher once said that “there is nothing permanent except change,” and Chemistry 12 will open our eyes to that continual chemical change all around us and how it leads to a fascinating and responsive state called equilibrium.
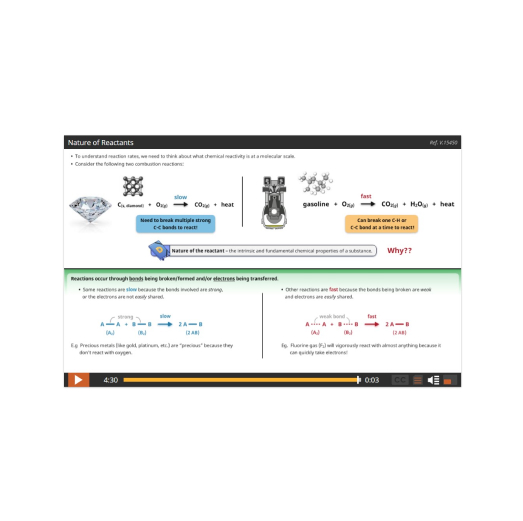
The course begins with a study of Reaction Kinetics, the factors that control the speed of a chemical reaction – from slow rusting to fast gasoline combustion – and why they have different speeds. From there we’ll discover that reactions can go forwards AND backwards (reversible reactions) and learn about the interesting properties and significance of these systems in studying Dynamic Equilibrium and Solubility Equilibrium, such as how billions more people on our planet have food to eat each day!
This understanding of chemical equilibrium then extends into the Theory and Applications of Acids and Bases, including why humans are able to avoid the otherwise “lethal effects” of acidic foods like lemons and pineapples due to the acid-base equilibria in our bodies — it’s true! And finally, we delve into Electrochemistry and discover how batteries actually work and why bananas turn brown (you’ve always wanted to know that, haven’t you?!) to eat each day!
Thankfully, the overarching theme of equilibrium will be there to guide us through every step of the way and provide the foundation we need to help us more deeply understand the chemical phenomena of the amazing world all around us!
The course begins with a study of Reaction Kinetics, the factors that control the speed of a chemical reaction – from slow rusting to fast gasoline combustion – and why they have different speeds. From there we’ll discover that reactions can go forwards AND backwards (reversible reactions) and learn about the interesting properties and significance of these systems in studying Dynamic Equilibrium and Solubility Equilibrium, such as how billions more people on our planet have food to eat each day!
This understanding of chemical equilibrium then extends into the Theory and Applications of Acids and Bases, including why humans are able to avoid the otherwise “lethal effects” of acidic foods like lemons and pineapples due to the acid-base equilibria in our bodies — it’s true! And finally, we delve into Electrochemistry and discover how batteries actually work and why bananas turn brown (you’ve always wanted to know that, haven’t you?!) to eat each day!
Thankfully, the overarching theme of equilibrium will be there to guide us through every step of the way and provide the foundation we need to help us more deeply understand the chemical phenomena of the amazing world all around us!
Table of Contents
*Each lesson is designed to take 60 – 90 minutes to complete with the exception of major projects and assignments.
Lesson 1: Chemical Reactions: From Blazing Fast to Slower than Molasses
Lesson 2: Can Chemical Reactions have Speedometers?
Lesson 3: Factors Affecting Reaction Rates
Lesson 4: Collision Theory
Lesson 5: How Energy Affects Reaction Rates
Lesson 6: Looking Inside Reactions
Lesson 7: (Optional) Introduction to Rate Law Expressions
Lesson 8: Reaction Kinetics Unit Summary
Lesson 2: Can Chemical Reactions have Speedometers?
Lesson 3: Factors Affecting Reaction Rates
Lesson 4: Collision Theory
Lesson 5: How Energy Affects Reaction Rates
Lesson 6: Looking Inside Reactions
Lesson 7: (Optional) Introduction to Rate Law Expressions
Lesson 8: Reaction Kinetics Unit Summary
Lesson 1: Dynamic Chemical Equilibrium: What is it?
Lesson 2: Dynamic Equilibrium: Why is it?
Lesson 3: Le Chatelier’s Principle!
Lesson 4: Le Chatelier’s Principle in Action
Lesson 5: Introducing the Equilibrium Constant, Keq
Lesson 6: Calculations with Keq
Lesson 7: Tools to Solve Equilibrium Problems: Q-Values and the ICE Box
Lesson 8: Dynamic Equilibrium Summary
Lesson 2: Dynamic Equilibrium: Why is it?
Lesson 3: Le Chatelier’s Principle!
Lesson 4: Le Chatelier’s Principle in Action
Lesson 5: Introducing the Equilibrium Constant, Keq
Lesson 6: Calculations with Keq
Lesson 7: Tools to Solve Equilibrium Problems: Q-Values and the ICE Box
Lesson 8: Dynamic Equilibrium Summary
Lesson 1: Know Your Solutions: Iconic and Molecular
Lesson 2: What is Solubility?
Lesson 3: The Power of Predicting Solubility
Lesson 4: Using the Solubility Product Constant Ksp
Lesson 5: An Old Friend: Q-Values and Solubility
Lesson 6: Le Chatelier, Solubility, Common Ions, and Temperature
Lesson 7: Solubility Equilibria Unit Summary
Lesson 2: What is Solubility?
Lesson 3: The Power of Predicting Solubility
Lesson 4: Using the Solubility Product Constant Ksp
Lesson 5: An Old Friend: Q-Values and Solubility
Lesson 6: Le Chatelier, Solubility, Common Ions, and Temperature
Lesson 7: Solubility Equilibria Unit Summary
Lesson 1: What are Acids & Bases, Anyways?
Lesson 2: An Even Better Theory: Brønsted Acids & Bases
Lesson 3: Acids and Bases of All Types: Amphiprotic, Polyprotic, Weak, and Strong
Lesson 4: Acid/Base Equilibria: Intro to Ka, Kb, and Kw
Lesson 5: Finding Ion Concentrations in Acid/Base Solutions
Lesson 6: Determining Kb and Keq Values
Lesson 7: Logarithms and the Famous pH Scale
Lesson 8: Friends of pH: pOH and pKw
Lesson 9: Advanced Problems with pH, pOH, Kw, Ka, and Kb
Lesson 10: Theory of Acids and Bases Unit Summary
Lesson 2: An Even Better Theory: Brønsted Acids & Bases
Lesson 3: Acids and Bases of All Types: Amphiprotic, Polyprotic, Weak, and Strong
Lesson 4: Acid/Base Equilibria: Intro to Ka, Kb, and Kw
Lesson 5: Finding Ion Concentrations in Acid/Base Solutions
Lesson 6: Determining Kb and Keq Values
Lesson 7: Logarithms and the Famous pH Scale
Lesson 8: Friends of pH: pOH and pKw
Lesson 9: Advanced Problems with pH, pOH, Kw, Ka, and Kb
Lesson 10: Theory of Acids and Bases Unit Summary
Lesson 1: Acid/Base Indicators
Lesson 2: Acid/Base Titrations
Lesson 3: Hydrolysis of Salts
Lesson 4: Buffers
Lesson 5: Acids, Bases, and the Environment
Lesson 6: Applications of Acids and Bases Unit Summary
Lesson 2: Acid/Base Titrations
Lesson 3: Hydrolysis of Salts
Lesson 4: Buffers
Lesson 5: Acids, Bases, and the Environment
Lesson 6: Applications of Acids and Bases Unit Summary
Lesson 1: Oxidation and Reduction
Lesson 2: Identifying Redox Reactions
Lesson 3: Balancing Redox Reactions
Lesson 4: Predicting the Spontaneity of Redox Reactions
Lesson 5: Redox Titrations
Lesson 6: The Electrochemical Cell
Lesson 7: Redox Chemistry Applied: Batteries, Fuel Cells, Corrosion
Lesson 8: Electrolysis and its Applications
Lesson 9: Electrochemistry Unit Summary
Lesson 2: Identifying Redox Reactions
Lesson 3: Balancing Redox Reactions
Lesson 4: Predicting the Spontaneity of Redox Reactions
Lesson 5: Redox Titrations
Lesson 6: The Electrochemical Cell
Lesson 7: Redox Chemistry Applied: Batteries, Fuel Cells, Corrosion
Lesson 8: Electrolysis and its Applications
Lesson 9: Electrochemistry Unit Summary
Experience a lesson as your students would
Course Features
- Using chemistry, students will take on the role of a forensic scientist in order to solve a crime
- Real world examples such as energy changes in rocket fuel
- Students will research where organics exist in their home