Science 10
Earth Science: Do you ever wonder how the universe began? How do scientists propose its beginning, how do other historical models compare to what we understand today? And what do models have to do with any of it? Join us as we get hands on, creating models, understanding models, and reading about models in relation to space, history and many different aspects of science all while learning about different objects in space and their relation and effects on Earth.
Biology: Do you wonder why things in nature are the way they are? Do you wonder why some plants or animals can live in hot, humid areas of the world and some live in cool, dry areas? Genetics are the foundation for the diversity of all living things and in this course, you’ll set off on a virtual Student Exchange Program to Brazil to find out more! You’ll learn from the locals how the topic of genetics impacts gardens, farms and the Amazon Rainforest. You’ll also learn about students’ various local concerns and how they’re getting involved to take action! After touring the country, marvelling at the rich diversity, you’ll “travel” home to research and create an action plan of your own!
Chemistry: Flux, motion, movement, alteration, transformation. All these words apply to matter. Matter is dynamic and can undergo dramatic changes! Carbon dioxide and water undergo a chemical change to produce wood, leaves, fruit and much more, and nitrogen and hydrogen can be reacted industrially to produce the fertilizer needed to feed billions of people! Is chemical change random? How can we make sense of it and use chemical change to our advantage? We’ll be looking at this question from the perspective of modern atomic theory, chemical kinetics, and thermodynamics. Atoms are in a sort of dance, able to switch their partners to form new molecules, which can switch atoms with other molecules to make other new molecules! In this module, you’ll learn about different types of chemical reactions you experience every day, including brushing your teeth and nuclear fusion (believe it or not), and lay a solid foundation for chemistry literacy in a reactive world.
Physics: Energy surrounds us, everywhere! It’s in the lifting of a book, the turning of a dial, or the spinning of a wheel. It’s in the motion of walking down the street or the dropping of a ball. In this module we dive deep into the relationship between different types of energy, namely potential energy and kinetic energy, and inquire as to how energy is present in some of the most common things around us! Together we will build a book that outlines how energy is conserved in ordinary objects, like the piece of paper or screen you are looking at now!
Table of Contents
Lesson 2: Force and Work
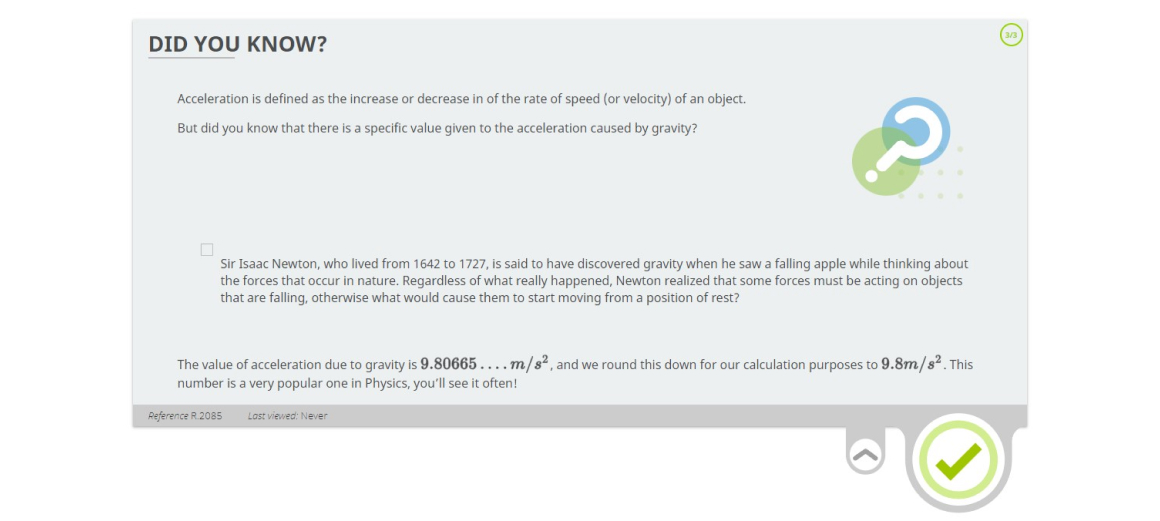
Lesson 3: Acceleration and Velocity – What’s the Difference?
Lesson 4: Potential Energy
Lesson 5: Potential Energy Continued
Lesson 6: Elastic Potential Energy
Lesson 7: Kinetic Energy
Lesson 8: Kinetic Energy Continued
Lesson 9: Energy A-Z: Research Your Topic
Lesson 10: Law of Conservation of Energy
Lesson 11: Law of Conservation of Energy Continued
Lesson 12: Heat
Lesson 13: Energy A-Z: Scientific Illustrations
Lesson 14: Energy Transformations in Daily Life
Lesson 15: Energy Transformations and Impacts
Lesson 16: Energy A-Z: Rough Draft
Lesson 17: First Nations Perspectives
Lesson 18: Energy A-Z: Wrap Up
Project: Energy A to Z
Project
Lesson 2: DNA Crash Course
Lesson 3: Departure
Lesson 4: Garden
Lesson 5: Punnett Squares
Lesson 6: Farm Tours
Lesson 7: Garden Design
Lesson 8: Artificial Selection
Lesson 9: Genetics
Lesson 10: Genetic Mutations
Lesson 11: Art of Reasoning Revision
Lesson 12: Adaptations and Natural Selection
Lesson 13: Rainforest
Lesson 14: Local Concerns
Lesson 15: Local Concern Action Steps
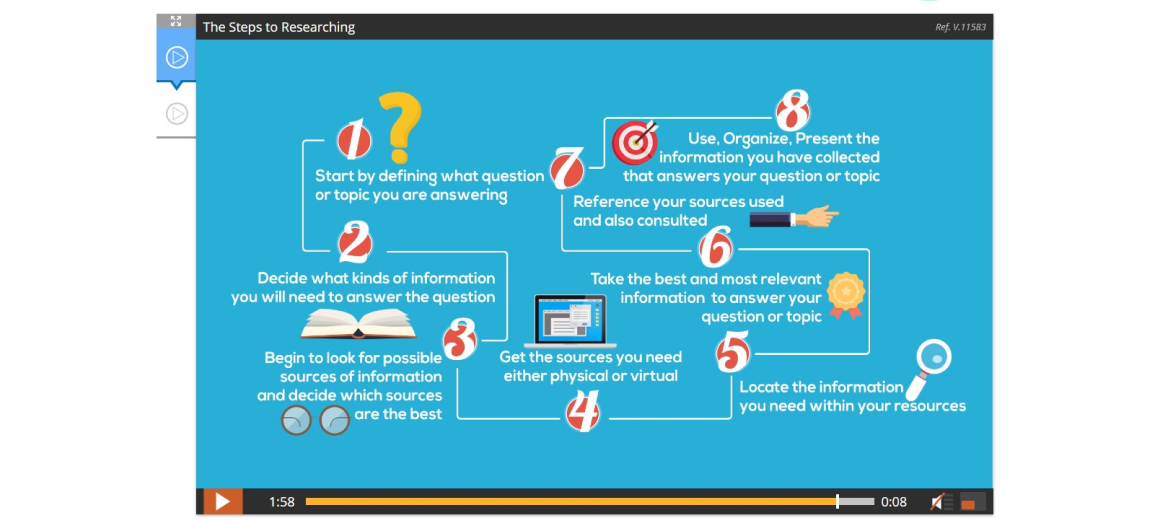
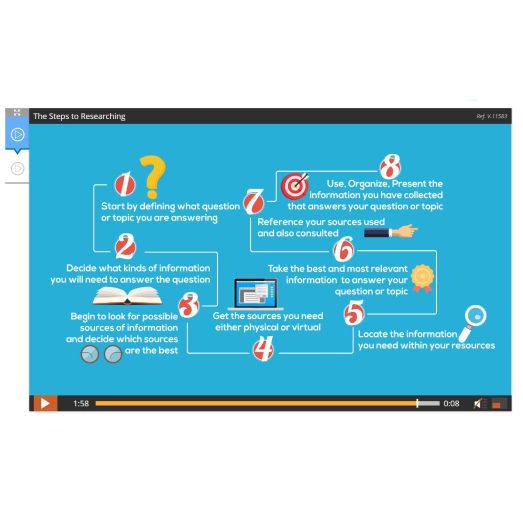
Lesson 16: Research Project – Gathering Information
Lesson 17: Research Project – Organizing Information
Lesson 18: Research Project – Writing Final Project
Lesson 19: Presentation
Project: Travel Journal
Project: Garden Project
Project: Action Plan Project
Lesson 2: Theories vs Facts and Laws
Lesson 3: Origin Stories
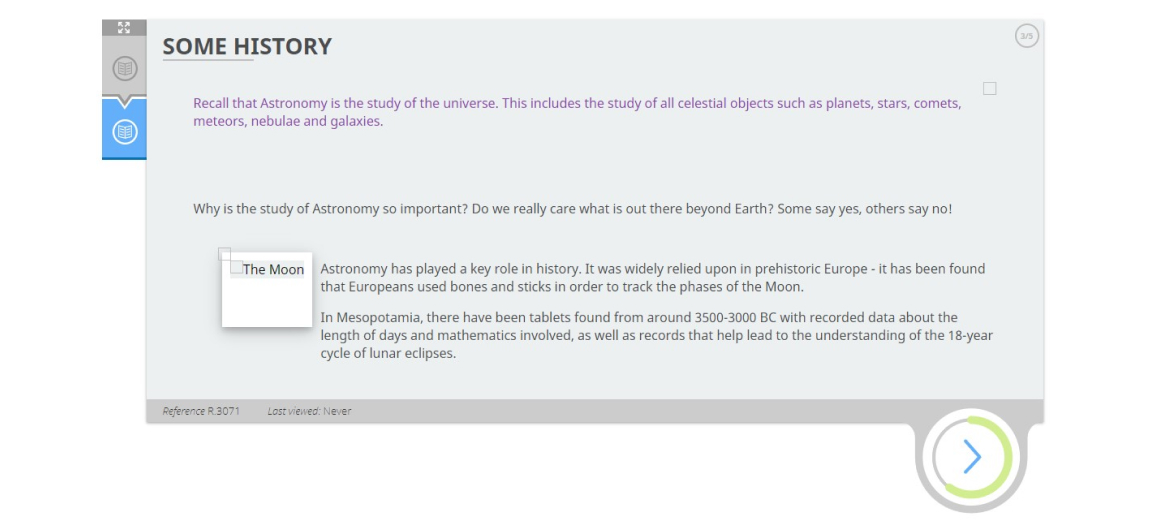
Lesson 4: Astronomy
Lesson 5: Scientific Models
Lesson 6: The Big Bang Theory
Lesson 7: The Doppler Effect
Lesson 8: Early Models of the Universe
Lesson 9: The Stars
Lesson 10: The Solar System I
Lesson 11: The Solar System II
Lesson 12: The Sun
Lesson 13: 3D Model I
Lesson 14: 3D Model II
Lesson 15: Technology and Astronomy
Lesson 16: Space Technology
Lesson 17: Space Exploration
Lesson 18: Technology Model
Project: Models
Lesson 2: Valence Electrons = Chemistry
Lesson 3: Covalent and Ionic Compounds
Lesson 4: Drawing Molecules with Lewis Structures
Lesson 5: Balancing Chemical Equations
Lesson 6: Chemical Reactions I
Lesson 7: Chemical Reactions II
Lesson 8: Intro to Acids and Bases
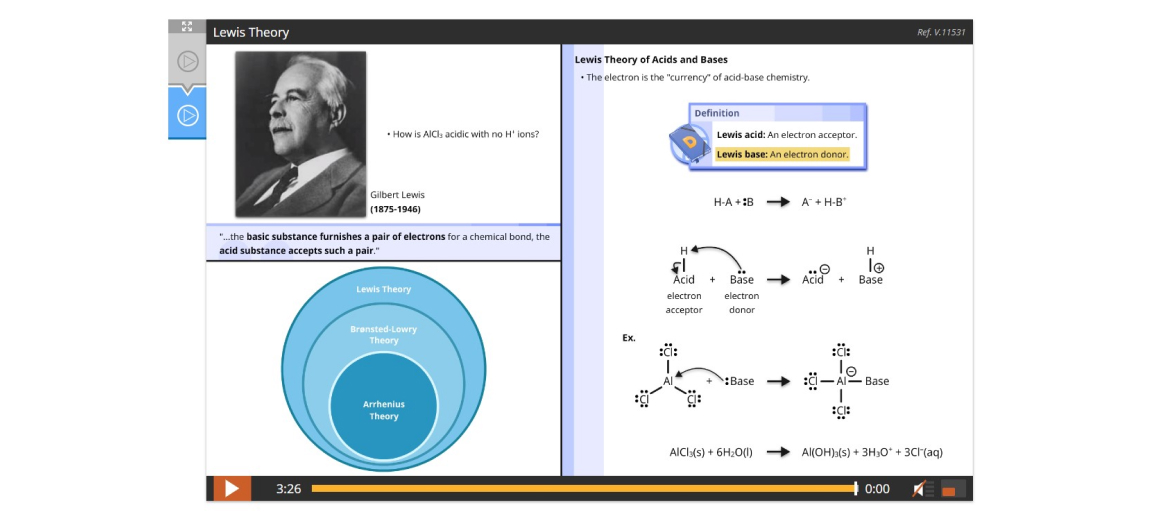
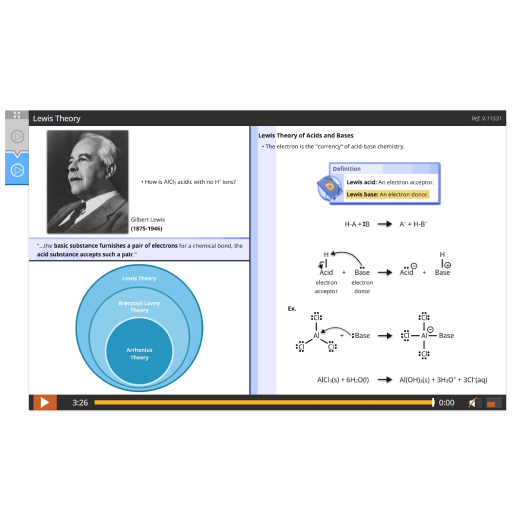
Lesson 9: Brønsted-Lowry and Lewis Theories
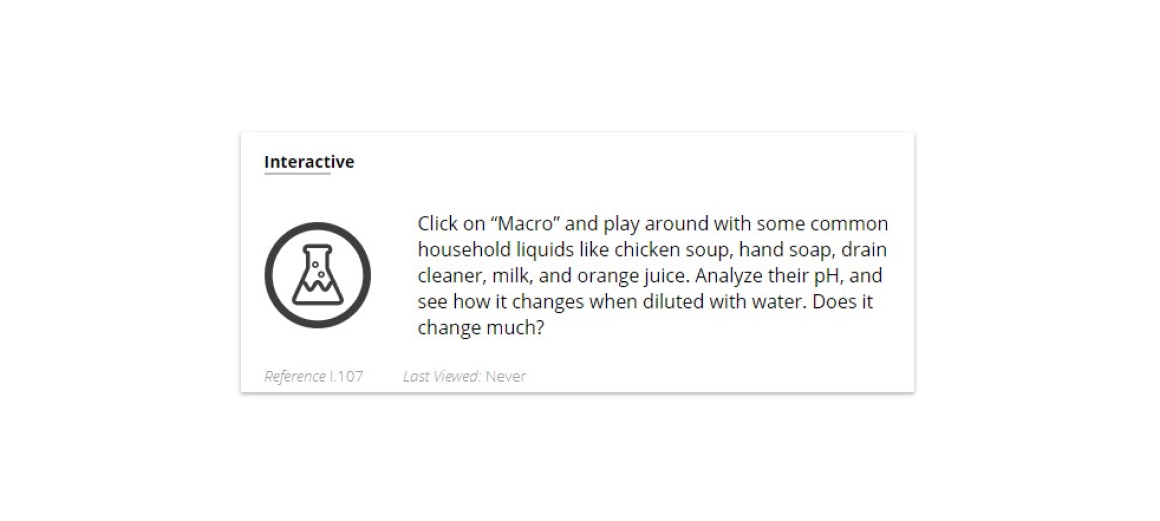
Lesson 10: pH and Molecular Structure
Lesson 11: Acids, Bases, and Your Teeth
Lesson 12: Acid-Base Indicators
Lesson 13: Chemical Energetics I
Lesson 14: Chemical Energetics II
Lesson 15: Factors Affecting the Rate of Reaction
Lesson 16: Experimenting with Reaction Rates
Lesson 17: Radiation
Lesson 18: Half-Life
Lesson 19: Nuclear Chemistry: Fission and Fusion
Project: Dental Hygiene Project
Experience a lesson as your students would
Course Features
- Using real world examples such as bungee jumping, roller coasters and jello students will learn about energy
- Going on a Virtual Student Exchange Program to Brazil to learn about genetic diversity
- Students will explore the origins of the universe