Chemistry 11
In this course students will be studying the physical and chemical properties of elements and compounds. It begins with a study of atomic theory in relation to quantum theory, the shape of molecules in 3D and periodic trends. The atomic and molecular structure of matter is analyzed through experimentation with emphasis on chemical reactions. Students will also be introduced to the concept of a mole and learn about stoichiometry. Solution chemistry (solubility, pH and titrations) and organic chemistry (naming compounds, functional groups and synthesis) are also covered.
These topics are examined using a wide variety of techniques including laboratory activities, analysis, projects and investigations.
A strong background in the chemistry topics from Science 9 and 10 as well as math skills is required.
Table of Contents
Lesson 2: Atomic Theory up to Bohr
Lesson 3: Intro to Quantum Theory
Lesson 4: Quantum Theory + The Periodic Table = A Perfect Fit!
Lesson 5: Periodic Trends
Lesson 6: Atoms Sharing Electrons: Electronegativity and Bonding
Lesson 7: Drawing Lewis Structures
Lesson 8: VSEPR Theory: Molecules in 3D!
Lesson 9: Sticky Molecules: Polarity and Intermolecular Forces
Project: Glossary
Lesson 2: Significant Figures: Understanding and Using Measured Numbers
Lesson 3: Avogadro’s Hypothesis and the Mole
Lesson 4: Calculations Using the Mole
Lesson 5: Molecular and Empirical Formulae (Enrichment – Optional)
Lesson 6: The Various Gas Laws
Lesson 7: The Ideal Gas Law
Lesson 8: Advanced Stoichiometry Calculations
Lesson 2: Percent Yield and Purity (Enrichment – Optional)
Lesson 3: A Review of Naming Inorganic Compounds
Lesson 4: Chemical Reaction Types: Part 1
Lesson 5: Chemical Reaction Types: Part 2
Lesson 6: Enthalpy Changes and Potential Energy Diagrams
Lesson 7: Enthalpy Changes from Bond Making and Breaking
Lesson 8: Green Chemistry
Lesson 2: What happens when a solid dissolves?
Lesson 3: Predicting Solubility (Enrichment – Optional)
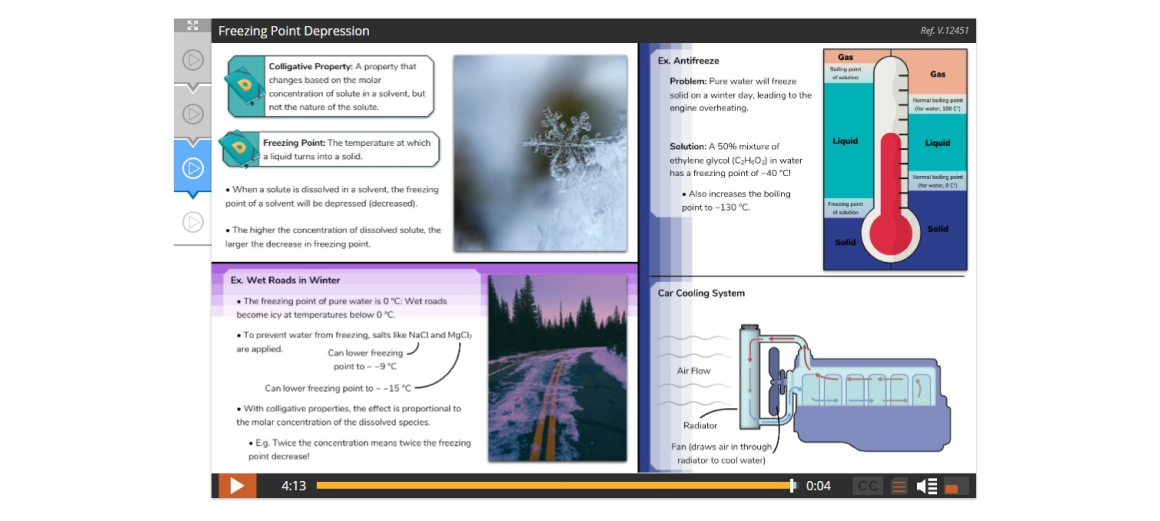
Lesson 4: Properties of Solutions
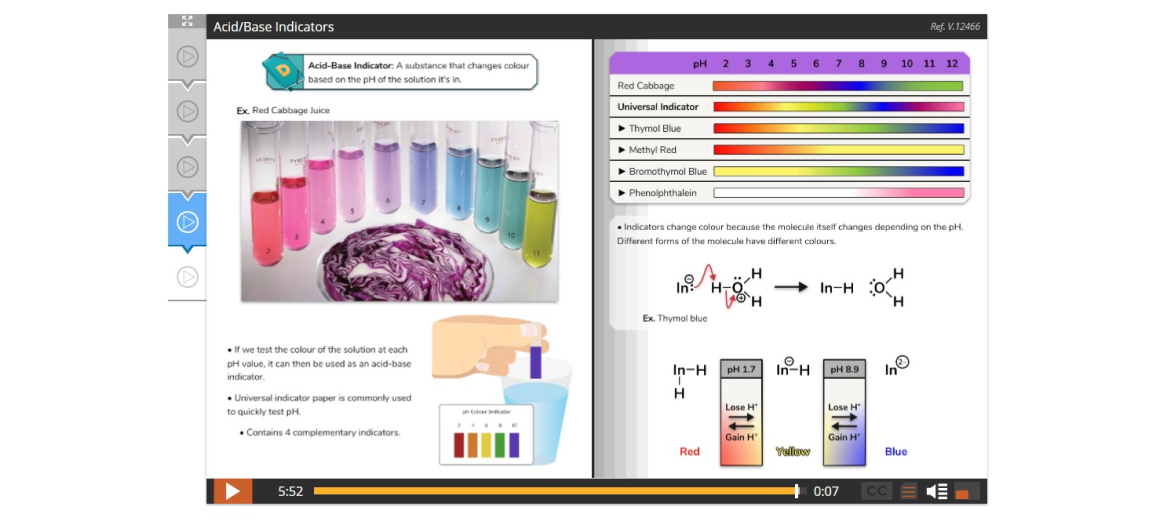
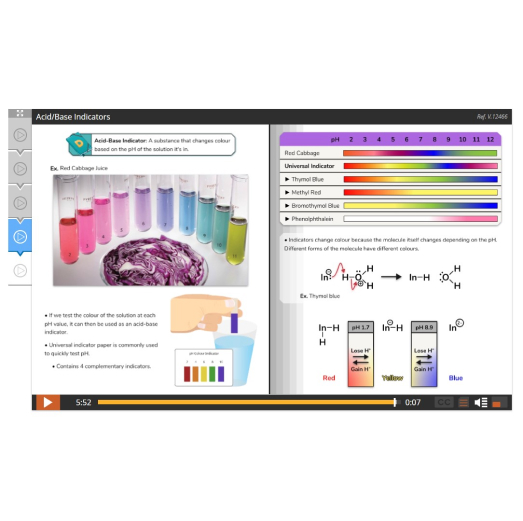
Lesson 5: Acids, Bases, and pH
Lesson 6: The Chemistry of Environmental Analysis
Lesson 2: How to Represent Molecules
Lesson 3: Isomerism and the Fundamental Functional Group: Alkanes
Lesson 4: Functional Groups: Alkenes and Alkynes
Lesson 5: Functional Groups: Alcohols, Ethers, Aldehydes, and Ketones
Lesson 6: Functional Groups: Carboxylic Acids, Esters, Amines, and Amides
Lesson 7: Intro to Organic Synthesis
Lesson 8: Organic Synthesis (Enrichment – Optional)
Experience a lesson as your students would
Course Features
- Using chemistry, students will take on the role of a forensic scientist in order to solve a crime
- Real world examples such as energy changes in rocket fuel
- Students will research where organics exist in their home